-
Chirality transfer from gold nanocluster to adsorbate evidenced by vibrational circular dichroism
I. Dolamic, B. Varnholt and T. Bürgi
Nature Communications, 6 (2015), p7117


DOI:10.1038/ncomms8117 | unige:98055 | Abstract | Article HTML | Article PDF | Supporting Info
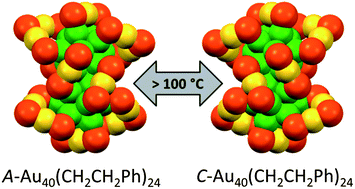
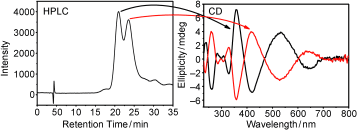
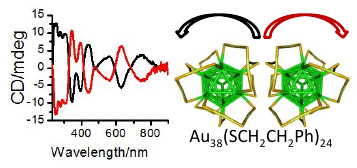
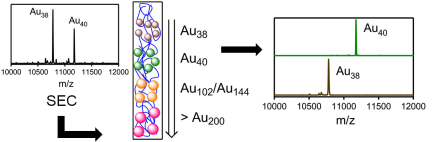
The transfer of chirality from one set of molecules to another is fundamental for applications in chiral technology and has likely played a crucial role for establishing homochirality on earth. Here we show that an intrinsically chiral gold cluster can transfer its handedness to an achiral molecule adsorbed on its surface. Solutions of chiral Au38(2-PET)24 (2-PET=2-phenylethylthiolate) cluster enantiomers show strong vibrational circular dichroism (VCD) signals in vibrations of the achiral adsorbate. Density functional theory (DFT) calculations reveal that 2-PET molecules adopt a chiral conformation. Chirality transfer from the cluster to the achiral adsorbate is responsible for the preference of one of the two mirror images. Intermolecular interactions between the adsorbed molecules on the crowded cluster surface seem to play a dominant role for the phenomena. Such chirality transfer from metals to adsorbates likely plays an important role in heterogeneous enantioselective catalysis.